Global Incidence of Pancreatic Cancer
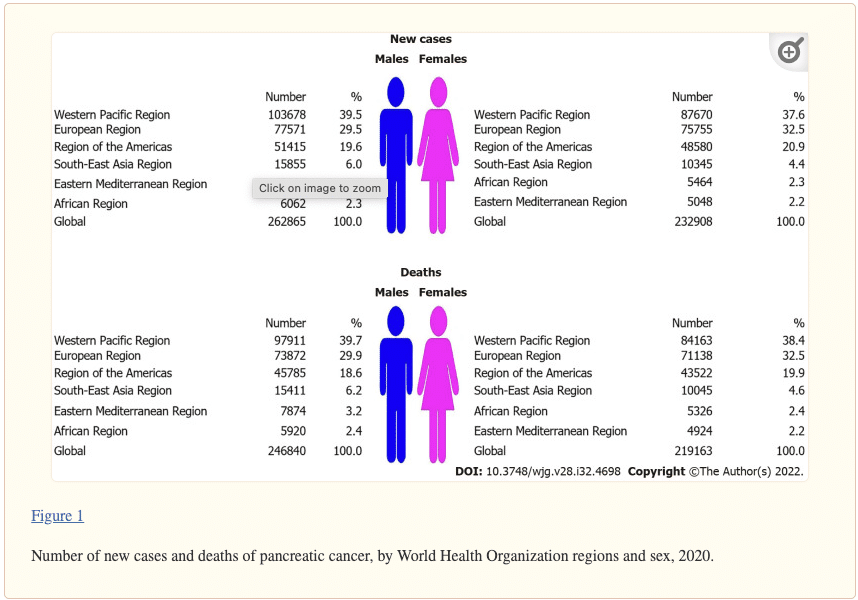
Pancreatic cancer is the 12th deadliest form of cancer, with a 5-year survival rate of just 10%, with more than half of the cases being diagnosed at an advanced stage. A total of 495773 (262865 male and 232908 female) new cases and 466003 (246840 male and 219163 female) deaths from pancreatic cancer were reported worldwide in 2020. In both sexes, most of the new cases (191348; 38.6% of the total) and deaths (182074; 39.1% of the total) occurred in the Western Pacific Region. In both sexes, the highest ASRs were found in the European Region, while the lowest rates were reported in the South-East Asia Region. The general pattern of rising pancreatic cancer incidence and mortality was seen across countries worldwide in observed period. Out of all countries with an increase in pancreatic cancer incidence, females in France and India showed the most marked rise in incidence rates (AAPC = +3.9% and AAPC = +3.7%, respectively). Decreasing incidence trends for pancreatic cancer were observed in some countries, but without significance. Out of all countries with an increase in pancreatic cancer mortality rates, Turkmenistan showed the most marked rise both in males (AAPC = +10.0%, 95%CI: 7.4–12.5) and females (AAPC = +6.4%, 95%CI: 3.5–9.5). The mortality trends of pancreatic cancer decreased in both sexes only in Canada and Mexico.
World Health Organization (WHO) estimated pancreatic cancer as the third leading cancer-related cause of death in people of all ages for both sexes in the USA, Germany, Italy, Austria, Czechia, Finland, Hungary, Malta, Spain, and Switzerland in 2020.In four countries (Finland, Qatar, Benin and Guadeloupe), pancreatic cancer was the second leading cause of death of all cancers in 2020 in females aged ≥ 70 years.
The most significant increase in pancreatic cancer incidence was noted in people aged ≥ 70 years in France in both males (by +4.2% per year) and females (by +4.9%). An increase was observed in most of the countries in both sexes for persons aged ≥ 50 years. A decreasing trend in the incidence of pancreatic cancer was recorded only in females aged ≥ 70 years in Canada (by 0.3% per year). Also, a decreasing trend in pancreatic cancer incidence in males aged ≥ 70 years was noted only in Canada (by 0.4% per year). In males aged 30–49 years, incidence rates decreased mainly in transitional countries (Czechia by 1.9%, Estonia 2.1%, Latvia 2.2%, Lithuania 2.2% and Slovakia 1.5%). In almost all countries, a significantly increasing trend in mortality of pancreatic cancer was recorded both in males and females aged ≥ 70 years.
To date, the causes of pancreatic carcinoma are still insufficiently known. However, certain risk factors have been identified, such as tobacco smoking, diabetes mellitus, obesity, dietary factors, alcohol abuse, age, ethnicity, family history and genetic factors, Helicobacter pylori infection, non-O blood group and chronic pancreatitis. In the general population, screening of large groups is not considered useful to detect the disease at its early stage, although newer techniques and the screening of tightly targeted groups (especially of those with family history), are being evaluated. Primary prevention is considered of utmost importance.

Based on the clinical stage of the tumor, pancreatic cancer is classified into four types: I (no spread or resectable), the cancer is limited to the pancreas and has grown 2 cm (IA) or greater than 2 cm but less than 4 cm (IB); II (local spread or borderline resectable), the cancer is > 4 cm and is limited to the pancreas, or there is spread locally to the nearby lymph nodes; III (wider spread or unresectable), cancer may have expanded to the nearby blood vessels or nerves, but has not metastasized to distant sites; IV (metastatic), cancer has spread to distant organs. Because pancreatic adenocarcinoma and the other less common exocrine cancers are typically diagnosed at a late stage (III or IV), it has a very poor prognosis compared to PanNET. At its early stages, pancreatic cancer usually lacks symptoms [7]. Upon progression of the tumor, it manifests as a gradual onset of non-specific symptoms including jaundice, weight loss, light-colored stools, abdominal pain, and fatigue [8].
Alcohol

A recent study found that heavy alcohol consumption was associated with a significant increase in pancreatic cancer risk among current smokers (age-adjusted odds ratio (OR) = 4.04, 95% CI: 1.58 – 10.37), whereas it was not observed among non-smokers (age-adjusted OR = 2.01, 95% CI: 0.50 – 8.18). Furthermore, low-to-moderate alcohol intake was associated with increased pancreas cancer risk among current smokers [56], suggesting that smoking can modify the alcohol-cancer relationship. However, the association between alcohol and smoking is very close. Therefore, it may be challenging to implicate alcohol as an independent risk factor for pancreatic cancer.
Obesity
Obesity is associated with an increased risk for several types of cancer including pancreatic cancer [57]. Some studies found that obesity increases the incidence and mortality of pancreatic cancer [58, 59]. A study by Li et al [60] found that being overweight (body mass index (BMI): 25.0 – 29.9 kg/m2) or obese (BMI ≥ 30 kg/m2) during early adulthood is associated with a higher risk of pancreatic cancer. Furthermore, obesity at an older age (30 – 79 years) was associated with lower overall survival.
According to an American Cancer Society (ACR) study, in both sexes, the risk of pancreatic cancer among obese was higher (RR = 2.08) compared to people of healthy BMI (18.5 – 24.9 kg/m2) [59]. A recent meta-analysis has confirmed the hypothesis that both general and abdominal fatness is associated with increased pancreatic cancer risk [61]. Besides, physical inactivity (which can contribute to fat accumulation and overweight) has been linked to an increased risk of pancreatic cancer.

It seems reasonable that diet would affect the risk of different digestive diseases and cancers, including those of the pancreas. Dietary factors impact up to 30-50% of pancreatic cancer, and there is evidence that certain foods are associated with higher risk, while others are even protective [42, 62, 63].
Consumption of red meats (especially when cooked at high temperature), processed meats, cholesterol, fried foods, and other foods containing nitrosamines may increase the risk of pancreatic cancer [64, 65]. It is possible that carcinogens in meat and nitrite or N-nitroso compounds that are used for preserving processed meats are involved in pancreatic cancer [66]. The results of a meta-analysis that included 11 case-control studies showed that red meat intake increased the pancreatic cancer risk by about 48% (95% CI: 1.25 – 1.76). On the other hand, a high intake of vegetables and fruits, especially those enriched in citrus and antioxidants, has a protective action, decreasing the risk by 38% (95% CI: 0.54 – 0.73) and 29% (95% CI: 0.59 – 0.84), respectively [67].
Also, another meta-analysis of 11 prospective studies found a positive association between pancreatic cancer incidence and high consumption of red (120 g/day) or processed meat (50 g/day) (RR = 1.13 and RR = 1.19 respectively) [68]. However, some studies have not supported these findings [69], or have provided support for the association among men only [70]. For example, the EPIC study found no association between pancreatic cancer risk and intake of red and processed meat, while poultry consumption was associated with an increased risk [71]. Interestingly, two studies reported that frequent nut consumption significantly lowers the risk of pancreatic cancer in women [72, 73]. Additionally, in a large UK cohort study in 2016, mortality for pancreatic cancer was lower for low meat-eaters (about 30-45% lower mortality), as well as vegetarians and vegans (about 50% lower mortality) compared with regular meat-eaters [74].
Occupational exposures
The etiological fraction of pancreatic cancer due to occupational exposures (involving exposure to metalworking and pesticides) within a population was estimated at 12%.
A meta-analysis of occupational exposures and pancreatic cancer reported an increased risk with nickel exposure [75]. However, in occupational settings, nickel may be associated with high concentrations of polychlorinated biphenyls, and the latter compounds could account for the observed increased risk [76, 77]. Carcinogenic mechanisms of nickel may include increasing DNA methylation, inhibiting DNA repair, and inducing apoptosis through the generation of reactive oxygen species [78–82].
Additionally, few studies have found a link between exposure to cadmium and arsenic and pancreatic cancer risk. Cadmium is a non-essential metal that is known to accumulate in the human pancreas increasing the risk and mortality of pancreatic cancer [83, 84]. Cadmium is a well-established carcinogen that acts on different steps of carcinogenesis, inhibiting DNA repair and causing genomic instability [85–87]. Furthermore, it causes transdifferentiation of pancreatic cells, inhibits DNA repair and induces or regulates the activity of several oncogenes or tumor-suppressor proteins that are expressed in human pancreatic cancer [83, 88, 89]. Arsenic exposure has been associated with increased cancer risk [90], but regarding its association with pancreatic cancer, little has been published. A potential link between childhood exposure to milk powder contaminated with arsenic and an almost two-fold excess mortality due to pancreatic cancer was recently reported [91, 92]. Inorganic arsenic is a highly toxic and carcinogenic metalloid, which can induce oxidative stress leading to inhibition of DNA repair [90, 93, 94] and DNA strand breaks as well as DNA adducts [95]. Moreover, alterations in the methylation status of oncogenes and tumor-suppressor genes, mediated by arsenic, may also play a role in carcinogenesis [96].
As opposite, selenium, which is an essential micronutrient [97, 98], has been inversely associated with several cancers including pancreatic [99–103], while only a small study published in 1989 showed an increased risk of pancreatic cancer due to high selenium levels [104]; however, no replication studies have been published. Aberrant expression patterns of some selenoproteins suggest that they are relevant in scavenging reactive oxygen species and diminishing oxidative damage [105, 106]. Also, selenium may boost p53 activity, leading to either DNA repair or apoptosis [107]. Selenium also seems to play a role as the antagonist of arsenic, cadmium, and lead, decreasing the oxidative stress caused by exposure to these elements [108, 109].
Pancreatic cancer is mostly diagnosed in an advanced stage, and 80-90% of patients have unresectable tumors at the moment of diagnosis. There are several reasons because this occurs.
First, early-stage pancreatic cancer is usually clinically silent, and most people who present with symptoms attributable to pancreatic cancer have advanced disease. Symptoms are non-specific and include abdominal pain, jaundice, pruritus, dark urine, and acholic stools, which may be presenting symptoms as a result of an obstruction within the biliary tree [195]. Furthermore, anorexia, weight loss (which can arise from anorexia), early satiety, dyspepsia, and nausea occur too [196], while less common manifestations include panniculitis and depression. Given the wide range of non-specific symptoms, there are a broad number of diseases that need to be differentiated [7], which include but are not limited to: cholangitis, cholecystitis, cholelithiasis, choledocholithiasis, choledochal cysts, duodenal or gastric ulcers, gastritis, pancreatitis, abdominal aortic aneurysm, lymphomas, and primary or secondary cancers of the biliary tree, liver, pancreas, stomach or intestine. Therefore, diagnosis can be delayed or missed, which makes pancreatic cancer the most common tumor detected at the autopsy studies [17, 24]. To date, there are several diagnostic tools available, such as abdominal ultrasonography, tri-phasic pancreatic-protocol CT (which is the standard for diagnosis and staging [197, 198]), magnetic resonance imaging (MRI) [7, 138] and endoscopic ultrasound-guided fine-needle aspiration for cytological diagnosis [7] (which sensitivity is reported to be about 80% [199]). Additionally, in symptomatic patients, measurement of blood levels of cancer antigen 19-9 can help to confirm the diagnosis and predict prognosis and recurrence after resection [200]; however, it cannot stand as an individual screening tool for asymptomatic patients because it is not tumor-specific [201]

Smoking
Over one thousand million people practice smoking of tobacco worldwide, and it represents the most important environmental factor for pancreatic cancer in the world. The International Agency for Research on Cancer has confirmed that smoking is causally associated with pancreatic cancer [13, 43]. The risk of pancreatic cancer increases with the duration of smoking and the number of cigarettes smoked daily. The risk is nearly two times higher in smokers than in non-smokers [44–46]; additionally, a recent meta-analysis of 82 studies found that the relative risk (RR) of pancreatic cancer was RR = 1.74 for current and RR = 1.2 for former smokers and the risk persists for at least 10 years after smoking cessation [47–49].
In 2012, the European Prospective Investigation into Cancer (EPIC) study showed that the risk of pancreatic cancer increases for every five cigarettes smoked per day, and also, passive smoking can increase the risk of pancreatic cancer by 50% [49, 50]. While smoking prevalence has declined in many developed countries, it remains high in others and is increasing among women and in developing countries. For example, in 2011, a study estimated that around 26.2% of pancreatic cancers in men and 31.0% in women were linked to tobacco smoking in the UK [20], while in the world’s two most populous nations, India and China, smoker users are home to more smokers than the entire population of Europe [51].
The risk of pancreatic cancer associated with smoking remains elevated after allowing for potential confounding factors such as alcohol consumption.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [PubMed] [CrossRef] [Google Scholar]
2. Ferlay J EM, Lam F, Colombet M, Mery L, Pineros M, Znaor A, Soerjomataram I, Global cancer observatory: cancer today. Lyon, France: International Agency for Research on Cancer; Available from: https://gco.iarc.fr/today, Accessed 05 October 2018. [Google Scholar]
3. Bosetti C, Lucenteforte E, Silverman DT, Petersen G, Bracci PM, Ji BT, Negri E. et al. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4) Ann Oncol. 2012;23(7):1880–1888. doi: 10.1093/annonc/mdr541. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
4. Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, Griffin C. et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64(7):2634–2638. doi: 10.1158/0008-5472.CAN-03-3823. [PubMed] [CrossRef] [Google Scholar]
5. Hidalgo M, Cascinu S, Kleeff J, Labianca R, Lohr JM, Neoptolemos J, Real FX. et al. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology. 2015;15(1):8–18. doi: 10.1016/j.pan.2014.10.001. [PubMed] [CrossRef] [Google Scholar]
6. Organization WH. World Cancer Report 2014. Accessed 06 October 2018. [Google Scholar]
7. De La Cruz MS, Young AP, Ruffin MT. Diagnosis and management of pancreatic cancer. Am Fam Physician. 2014;89(8):626–632. [PubMed] [Google Scholar]
8. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [PubMed] [CrossRef] [Google Scholar]
9. Mohammed S, Van Buren G 2nd, Fisher WE. Pancreatic cancer: advances in treatment. World J Gastroenterol. 2014;20(28):9354–9360. [PMC free article] [PubMed] [Google Scholar]
10. SEER Cancer Statistics Review, 1975-2013 [Internet]. National Cancer Institue, Bethesda, MD. 2016. Available from: https://seer.cancer.gov/csr/1975_2015/. Accessed 05 October 2018.
11. Malvezzi M, Carioli G, Bertuccio P, Rosso T, Boffetta P, Levi F, La Vecchia C. et al. European cancer mortality predictions for the year 2016 with focus on leukaemias. Ann Oncol. 2016;27(4):725–731. doi: 10.1093/annonc/mdw022. [PubMed] [CrossRef] [Google Scholar]
12. Bosetti C, Bertuccio P, Negri E, La Vecchia C, Zeegers MP, Boffetta P. Pancreatic cancer: overview of descriptive epidemiology. Mol Carcinog. 2012;51(1):3–13. doi: 10.1002/mc.20785. [PubMed] [CrossRef] [Google Scholar]
13. Ezzati M, Henley SJ, Lopez AD, Thun MJ. Role of smoking in global and regional cancer epidemiology: current patterns and data needs. Int J Cancer. 2005;116(6):963–971. doi: 10.1002/ijc.21100. [PubMed] [CrossRef] [Google Scholar]
14. Willett WC. Diet and cancer. Oncologist. 2000;5(5):393–404. doi: 10.1634/theoncologist.5-5-393. [PubMed] [CrossRef] [Google Scholar]
15. Genkinger JM, Spiegelman D, Anderson KE, Bernstein L, van den Brandt PA, Calle EE, English DR. et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int J Cancer. 2011;129(7):1708–1717. doi: 10.1002/ijc.25794. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
16. Jarosz M, Sekula W, Rychlik E. Influence of diet and tobacco smoking on pancreatic cancer incidence in poland in 1960-2008. Gastroenterol Res Pract. 2012;2012:682156. doi: 10.1155/2012/682156. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
17. Avgerinos DV, Bjornsson J. Malignant neoplasms: discordance between clinical diagnoses and autopsy findings in 3,118 cases. APMIS. 2001;109(11):774–780. doi: 10.1034/j.1600-0463.2001.d01-145.x. [PubMed] [CrossRef] [Google Scholar]
18. Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ. 2005;83(3):171–177. [PMC free article] [PubMed] [Google Scholar]
19. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D. et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. doi: 10.1016/j.ejca.2012.12.027. [PubMed] [CrossRef] [Google Scholar]
20. Parkin DM, Boyd L, Walker LC. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105(Suppl 2):S77–81. doi: 10.1038/bjc.2011.489. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
21. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [PubMed] [CrossRef] [Google Scholar]
22. Oberstein PE, Olive KP. Pancreatic cancer: why is it so hard to treat? Therap Adv Gastroenterol. 2013;6(4):321–337. doi: 10.1177/1756283X13478680. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
23. Levi F, Lucchini F, Negri E, La Vecchia C. Pancreatic cancer mortality in Europe: the leveling of an epidemic. Pancreas. 2003;27(2):139–142. doi: 10.1097/00006676-200308000-00006. [PubMed] [CrossRef] [Google Scholar]
24. Sens MA, Zhou X, Weiland T, Cooley AM. Unexpected neoplasia in autopsies: potential implications for tissue and organ safety. Arch Pathol Lab Med. 2009;133(12):1923–1931. [PubMed] [Google Scholar]
25. Lambe M, Eloranta S, Wigertz A, Blomqvist P. Pancreatic cancer; reporting and long-term survival in Sweden. Acta Oncol. 2011;50(8):1220–1227. doi: 10.3109/0284186X.2011.599338. [PubMed] [CrossRef] [Google Scholar]
26. Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22(44):9694–9705. doi: 10.3748/wjg.v22.i44.9694. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
27. Wu W, He X, Yang L, Wang Q, Bian X, Ye J, Li Y. et al. Rising trends in pancreatic cancer incidence and mortality in 2000-2014. Clin Epidemiol. 2018;10:789–797. doi: 10.2147/CLEP.S160018. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
28. Ferlay J EM, Lam F, Colombet M, Mery L, Pineros M, Znaor A, Soerjomataram I, Global Cancer Observatory: Cancer Tomorrow. Lyon, France: International Agency for Research on Cancer; Accessed 07 October 2018. Available from: http://gco.iarc.fr/tomorrow/graphic-isotype?type=1&population=900&mode=population&sex=0&cancer=39&age_group=value&apc_male=0&apc_female=0. [Google Scholar]
29. Weiss W, Benarde MA. The temporal relation between cigarette smoking and pancreatic cancer. Am J Public Health. 1983;73(12):1403–1404. doi: 10.2105/AJPH.73.12.1403. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
30. Agarwal B, Correa AM, Ho L. Survival in pancreatic carcinoma based on tumor size. Pancreas. 2008;36(1):e15–20. doi: 10.1097/mpa.0b013e31814de421. [PubMed] [CrossRef] [Google Scholar]
31. United States National Cancer Institute. Pancreatic cancer: statistics 2018, May. Available from: https://www.cancer.net/cancer-types/pancreatic-cancer/statistics. Accessed 07 October 2018.
32. Rossi S, Baili P, Capocaccia R, Caldora M, Carrani E, Minicozzi P, Pierannunzio D. et al. The EUROCARE-5 study on cancer survival in Europe 1999-2007: Database, quality checks and statistical analysis methods. Eur J Cancer. 2015;51(15):2104–2119. doi: 10.1016/j.ejca.2015.08.001. [PubMed] [CrossRef] [Google Scholar]
33. Survival of cancer patients in Europe. European Cancer Registry (EUROCARE). http://www.eurocare.it. 2015, October. Accessed 05 October 2018.
34. Cancer Research UK, https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/pancreatic-cancer/survival#ref-2, Accessed October 20 2018.
35. Carrato A, Falcone A, Ducreux M, Valle JW, Parnaby A, Djazouli K, Alnwick-Allu K. et al. A Systematic Review of the Burden of Pancreatic Cancer in Europe: Real-World Impact on Survival, Quality of Life and Costs. J Gastrointest Cancer. 2015;46(3):201–211. doi: 10.1007/s12029-015-9724-1. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
36. Oh SY, Edwards A, Mandelson MT, Lin B, Dorer R, Helton WS, Kozarek RA. et al. Rare long-term survivors of pancreatic adenocarcinoma without curative resection. World J Gastroenterol. 2015;21(48):13574–13581. doi: 10.3748/wjg.v21.i48.13574. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
37. Dimastromatteo J, Houghton JL, Lewis JS, Kelly KA. Challenges of pancreatic cancer. Cancer J. 2015;21(3):188–193. doi: 10.1097/PPO.0000000000000109. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
38. Yamamoto T, Yagi S, Kinoshita H, Sakamoto Y, Okada K, Uryuhara K, Morimoto T. et al. Long-term survival after resection of pancreatic cancer: a single-center retrospective analysis. World J Gastroenterol. 2015;21(1):262–268. doi: 10.3748/wjg.v21.i1.262. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
39. American Cancer Society. https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html. Accessed 10 October 2018.
40. Karim-Kos HE, de Vries E, Soerjomataram I, Lemmens V, Siesling S, Coebergh JW. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. 2008;44(10):1345–1389. doi: 10.1016/j.ejca.2007.12.015. [PubMed] [CrossRef] [Google Scholar]
41. Brenner H, Hakulinen T. Population-based monitoring of cancer patient survival in situations with imperfect completeness of cancer registration. Br J Cancer. 2005;92(3):576–579. doi: 10.1038/sj.bjc.6602323. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
42. Midha S, Chawla S, Garg PK. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett. 2016;381(1):269–277. doi: 10.1016/j.canlet.2016.07.022. [PubMed] [CrossRef] [Google Scholar]
43. Iarc Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
44. Kuzmickiene I, Everatt R, Virviciute D, Tamosiunas A, Radisauskas R, Reklaitiene R, Milinaviciene E. Smoking and other risk factors for pancreatic cancer: a cohort study in men in Lithuania. Cancer Epidemiol. 2013;37(2):133–139. doi: 10.1016/j.canep.2012.10.001. [PubMed] [CrossRef] [Google Scholar]
45. Mizuno S, Nakai Y, Isayama H, Kawahata S, Saito T, Takagi K, Watanabe T. et al. Smoking, family history of cancer, and diabetes mellitus are associated with the age of onset of pancreatic cancer in Japanese patients. Pancreas. 2014;43(7):1014–1017. doi: 10.1097/MPA.0000000000000158. [PubMed] [CrossRef] [Google Scholar]
46. Pelucchi C, Galeone C, Polesel J, Manzari M, Zucchetto A, Talamini R, Franceschi S. et al. Smoking and body mass index and survival in pancreatic cancer patients. Pancreas. 2014;43(1):47–52. doi: 10.1097/MPA.0b013e3182a7c74b. [PubMed] [CrossRef] [Google Scholar]
47. Rastogi T, Devesa S, Mangtani P, Mathew A, Cooper N, Kao R, Sinha R. Cancer incidence rates among South Asians in four geographic regions: India, Singapore, UK and US. Int J Epidemiol. 2008;37(1):147–160. doi: 10.1093/ije/dym219. [PubMed] [CrossRef] [Google Scholar]
48. Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg. 2008;393(4):535–545. doi: 10.1007/s00423-007-0266-2. [PubMed] [CrossRef] [Google Scholar]
49. Vrieling A, Bueno-de-Mesquita HB, Boshuizen HC, Michaud DS, Severinsen MT, Overvad K, Olsen A. et al. Cigarette smoking, environmental tobacco smoke exposure and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2010;126(10):2394–2403. doi: 10.1002/ijc.24907. [PubMed] [CrossRef] [Google Scholar]
50. Lynch SM, Vrieling A, Lubin JH, Kraft P, Mendelsohn JB, Hartge P, Canzian F. et al. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol. 2009;170(4):403–413. doi: 10.1093/aje/kwp134. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
51. Tan D. Smoking in Asia: a looming health epidemic. Asian Scientist Magazine Read more from Asian Scientist Magazine at: https://wwwasianscientistcom/2012/08/features/smoking-in-asia-looming-health-epidemic-2012/. 2012. Accessed 05 October 2018.
52. Wang YT, Gou YW, Jin WW, Xiao M, Fang HY. Association between alcohol intake and the risk of pancreatic cancer: a dose-response meta-analysis of cohort studies. BMC Cancer. 2016;16:212. doi: 10.1186/s12885-016-2241-1. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
53. Lucenteforte E, La Vecchia C, Silverman D, Petersen GM, Bracci PM, Ji BT, Bosetti C. et al. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4) Ann Oncol. 2012;23(2):374–382. doi: 10.1093/annonc/mdr120. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
54. Tramacere I, Scotti L, Jenab M, Bagnardi V, Bellocco R, Rota M, Corrao G. et al. Alcohol drinking and pancreatic cancer risk: a meta-analysis of the dose-risk relation. Int J Cancer. 2010;126(6):1474–1486. doi: 10.1002/ijc.24936. [PubMed] [CrossRef] [Google Scholar]
55. Michaud DS, Vrieling A, Jiao L, Mendelsohn JB, Steplowski E, Lynch SM, Wactawski-Wende J. et al. Alcohol intake and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium (PanScan) Cancer Causes Control. 2010;21(8):1213–1225. doi: 10.1007/s10552-010-9548-z. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
56. Rahman F, Cotterchio M, Cleary SP, Gallinger S. Association between alcohol consumption and pancreatic cancer risk: a case-control study. PLoS One. 2015;10(4):e0124489. doi: 10.1371/journal.pone.0124489. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
57. Davoodi SH, Malek-Shahabi T, Malekshahi-Moghadam A, Shahbazi R, Esmaeili S. Obesity as an important risk factor for certain types of cancer. Iran J Cancer Prev. 2013;6(4):186–194. [PMC free article] [PubMed] [Google Scholar]
58. Berrington de Gonzalez A, Sweetland S, Spencer E. A meta-analysis of obesity and the risk of pancreatic cancer. Br J Cancer. 2003;89(3):519–523. doi: 10.1038/sj.bjc.6601140. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
59. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [PubMed] [CrossRef] [Google Scholar]
60. Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, Abbruzzese JL. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301(24):2553–2562. doi: 10.1001/jama.2009.886. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
61. Aune D, Greenwood DC, Chan DS, Vieira R, Vieira AR, Navarro Rosenblatt DA, Cade JE. et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23(4):843–852. doi: 10.1093/annonc/mdr398. [PubMed] [CrossRef] [Google Scholar]
62. Michaud DS, Skinner HG, Wu K, Hu F, Giovannucci E, Willett WC, Colditz GA. et al. Dietary patterns and pancreatic cancer risk in men and women. J Natl Cancer Inst. 2005;97(7):518–524. doi: 10.1093/jnci/dji094. [PubMed] [CrossRef] [Google Scholar]
63. Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: a summary review of meta-analytical studies. Int J Epidemiol. 2015;44(1):186–198. doi: 10.1093/ije/dyu240. [PubMed] [CrossRef] [Google Scholar]
64. Lightsey D. Comment on ‘Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies’. Br J Cancer. 2012;107(4):754–755. doi: 10.1038/bjc.2012.111. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
65. Stolzenberg-Solomon RZ, Cross AJ, Silverman DT, Schairer C, Thompson FE, Kipnis V, Subar AF. et al. Meat and meat-mutagen intake and pancreatic cancer risk in the NIH-AARP cohort. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2664–2675. doi: 10.1158/1055-9965.EPI-07-0378. [PubMed] [CrossRef] [Google Scholar]
66. Beaney AJ, Banim PJR, Luben R, Lentjes MAH, Khaw KT, Hart AR. Higher meat intake is positively associated with higher risk of developing pancreatic cancer in an age-dependent manner and are modified by plasma antioxidants: a prospective cohort study (EPIC-Norfolk) using data from food diaries. Pancreas. 2017;46(5):672–678. doi: 10.1097/MPA.0000000000000819. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
67. Paluszkiewicz P, Smolinska K, Debinska I, Turski WA. Main dietary compounds and pancreatic cancer risk. The quantitative analysis of case-control and cohort studies. Cancer Epidemiol. 2012;36(1):60–67. doi: 10.1016/j.canep.2011.05.004. [PubMed] [CrossRef] [Google Scholar]
68. Larsson SC, Wolk A. Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies. Br J Cancer. 2012;106(3):603–607. doi: 10.1038/bjc.2011.585. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
69. Miller PE, Alexander D. A review and meta-analysis of prospective studies of red and processed meat and pancreatic cancer. FASEB J. 2016;30:902–909. [Google Scholar]
70. Aschebrook-Kilfoy B, Cross AJ, Stolzenberg-Solomon RZ, Schatzkin A, Hollenbeck AR, Sinha R, Ward MH. Pancreatic cancer and exposure to dietary nitrate and nitrite in the NIH-AARP Diet and Health Study. Am J Epidemiol. 2011;174(3):305–315. doi: 10.1093/aje/kwr092. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
71. Rohrmann S, Linseisen J, Nothlings U, Overvad K, Egeberg R, Tjonneland A, Boutron-Ruault MC. et al. Meat and fish consumption and risk of pancreatic cancer: results from the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2013;132(3):617–624. doi: 10.1002/ijc.27637. [PubMed] [CrossRef] [Google Scholar]
72. Bao Y, Hu FB, Giovannucci EL, Wolpin BM, Stampfer MJ, Willett WC, Fuchs CS. Nut consumption and risk of pancreatic cancer in women. Br J Cancer. 2013;109(11):2911–2916. doi: 10.1038/bjc.2013.665. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
73. Wu L, Wang Z, Zhu J, Murad AL, Prokop LJ, Murad MH. Nut consumption and risk of cancer and type 2 diabetes: a systematic review and meta-analysis. Nutr Rev. 2015;73(7):409–425. doi: 10.1093/nutrit/nuv006. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
74. Appleby PN, Crowe FL, Bradbury KE, Travis RC, Key TJ. Mortality in vegetarians and comparable nonvegetarians in the United Kingdom. Am J Clin Nutr. 2016;103(1):218–230. doi: 10.3945/ajcn.115.119461. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
75. Ojajarvi IA, Partanen TJ, Ahlbom A, Boffetta P, Hakulinen T, Jourenkova N, Kauppinen TP. et al. Occupational exposures and pancreatic cancer: a meta-analysis. Occup Environ Med. 2000;57(5):316–324. doi: 10.1136/oem.57.5.316. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
76. Bosch de Basea M, Porta M, Alguacil J, Puigdomenech E, Gasull M, Garrido JA, Lopez T. et al. Relationships between occupational history and serum concentrations of organochlorine compounds in exocrine pancreatic cancer. Occup Environ Med. 2011;68(5):332–338. doi: 10.1136/oem.2009.054197. [PubMed] [CrossRef] [Google Scholar]
77. Porta M, Malats N, Jariod M, Grimalt JO, Rifa J, Carrato A, Guarner L. et al. Serum concentrations of organochlorine compounds and K-ras mutations in exocrine pancreatic cancer. PANKRAS II Study Group. Lancet. 1999;354(9196):2125–2129. doi: 10.1016/S0140-6736(99)04232-4. [PubMed] [CrossRef] [Google Scholar]
78. Hartwig A, Kruger I, Beyersmann D. Mechanisms in nickel genotoxicity: the significance of interactions with DNA repair. Toxicol Lett. 1994;72(1-3):353–358. doi: 10.1016/0378-4274(94)90048-5. [PubMed] [CrossRef] [Google Scholar]
79. Hartwig A, Mullenders LH, Schlepegrell R, Kasten U, Beyersmann D. Nickel(II) interferes with the incision step in nucleotide excision repair in mammalian cells. Cancer Res. 1994;54(15):4045–4051. [PubMed] [Google Scholar]
80. Kasprzak KS. The role of oxidative damage in metal carcinogenicity. Chem Res Toxicol. 1991;4(6):604–615. doi: 10.1021/tx00024a002. [PubMed] [CrossRef] [Google Scholar]
81. Lee YW, Klein CB, Kargacin B, Salnikow K, Kitahara J, Dowjat K, Zhitkovich A. et al. Carcinogenic nickel silences gene expression by chromatin condensation and DNA methylation: a new model for epigenetic carcinogens. Mol Cell Biol. 1995;15(5):2547–2557. doi: 10.1128/MCB.15.5.2547. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
82. Ahamed M, Akhtar MJ, Siddiqui MA, Ahmad J, Musarrat J, Al-Khedhairy AA, AlSalhi MS. et al. Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology. 2011;283(2-3):101–108. doi: 10.1016/j.tox.2011.02.010. [PubMed] [CrossRef] [Google Scholar]
83. Schwartz GG, Reis IM. Is cadmium a cause of human pancreatic cancer? Cancer Epidemiol Biomarkers Prev. 2000;9(2):139–145. [PubMed] [Google Scholar]
84. Kriegel AM, Soliman AS, Zhang Q, El-Ghawalby N, Ezzat F, Soultan A, Abdel-Wahab M. et al. Serum cadmium levels in pancreatic cancer patients from the East Nile Delta region of Egypt. Environ Health Perspect. 2006;114(1):113–119. [PMC free article] [PubMed] [Google Scholar]
85. Hartwig A. Mechanisms in cadmium-induced carcinogenicity: recent insights. Biometals. 2010;23(5):951–960. doi: 10.1007/s10534-010-9330-4. [PubMed] [CrossRef] [Google Scholar]
86. Schwerdtle T, Ebert F, Thuy C, Richter C, Mullenders LH, Hartwig A. Genotoxicity of soluble and particulate cadmium compounds: impact on oxidative DNA damage and nucleotide excision repair. Chem Res Toxicol. 2010;23(2):432–442. doi: 10.1021/tx900444w. [PubMed] [CrossRef] [Google Scholar]
87. Bertin G, Averbeck D. Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review) Biochimie. 2006;88(11):1549–1559. doi: 10.1016/j.biochi.2006.10.001. [PubMed] [CrossRef] [Google Scholar]
88. Candeias S, Pons B, Viau M, Caillat S, Sauvaigo S. Direct inhibition of excision/synthesis DNA repair activities by cadmium: analysis on dedicated biochips. Mutat Res. 2010;694(1-2):53–59. doi: 10.1016/j.mrfmmm.2010.10.001. [PubMed] [CrossRef] [Google Scholar]
89. Waalkes MP, Cherian MG, Ward JM, Goyer RA. Immunohistochemical evidence of high concentrations of metallothionein in pancreatic hepatocytes induced by cadmium in rats. Toxicol Pathol. 1992;20(3 Pt 1):323–326. doi: 10.1177/019262339202000302. [PubMed] [CrossRef] [Google Scholar]
90. Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. IARC Monogr Eval Carcinog Risks Hum Suppl. 1987;7:1–440. [PubMed] [Google Scholar]
91. Yorifuji T, Tsuda T, Doi H, Grandjean P. Cancer excess after arsenic exposure from contaminated milk powder. Environ Health Prev Med. 2011;16(3):164–170. doi: 10.1007/s12199-010-0182-x. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
92. Yorifuji T, Tsuda T, Grandjean P. Unusual cancer excess after neonatal arsenic exposure from contaminated milk powder. J Natl Cancer Inst. 2010;102(5):360–361. doi: 10.1093/jnci/djp536. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
93. Straif K, Benbrahim-Tallaa L, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V. et al. A review of human carcinogens – Part C: metals, arsenic, dusts, and fibres. Lancet Oncol. 2009;10(5):453–454. doi: 10.1016/S1470-2045(09)70134-2. [PubMed] [CrossRef] [Google Scholar]
94. Qin XJ, Hudson LG, Liu W, Ding W, Cooper KL, Liu KJ. Dual actions involved in arsenite-induced oxidative DNA damage. Chem Res Toxicol. 2008;21(9):1806–1813. doi: 10.1021/tx8001548. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
95. Pu YS, Jan KY, Wang TC, Wang AS, Gurr JR. 8-Oxoguanine DNA glycosylase and MutY homolog are involved in the incision of arsenite-induced DNA adducts. Toxicol Sci. 2007;95(2):376–382. doi: 10.1093/toxsci/kfl166. [PubMed] [CrossRef] [Google Scholar]
96. Reichard JF, Puga A. Effects of arsenic exposure on DNA methylation and epigenetic gene regulation. Epigenomics. 2010;2(1):87–104. doi: 10.2217/epi.09.45. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
97. Brown KM, Arthur JR. Selenium, selenoproteins and human health: a review. Public Health Nutr. 2001;4(2B):593–599. doi: 10.1079/PHN2001143. [PubMed] [CrossRef] [Google Scholar]
98. Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007;9(7):775–806. doi: 10.1089/ars.2007.1528. [PubMed] [CrossRef] [Google Scholar]
99. Amaral AF, Cantor KP, Silverman DT, Malats N. Selenium and bladder cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2407–2415. doi: 10.1158/1055-9965.EPI-10-0544. [PubMed] [CrossRef] [Google Scholar]
100. Bardia A, Tleyjeh IM, Cerhan JR, Sood AK, Limburg PJ, Erwin PJ, Montori VM. Efficacy of antioxidant supplementation in reducing primary cancer incidence and mortality: systematic review and meta-analysis. Mayo Clin Proc. 2008;83(1):23–34. doi: 10.4065/83.1.23. [PubMed] [CrossRef] [Google Scholar]
101. Zhuo H, Smith AH, Steinmaus C. Selenium and lung cancer: a quantitative analysis of heterogeneity in the current epidemiological literature. Cancer Epidemiol Biomarkers Prev. 2004;13(5):771–778. [PubMed] [Google Scholar]
102. Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for preventing gastrointestinal cancers. Cochrane Database Syst Rev. 2008;3:CD004183. doi: 10.1002/14651858.CD004183.pub3. [PubMed] [CrossRef] [Google Scholar]
103. Etminan M, FitzGerald JM, Gleave M, Chambers K. Intake of selenium in the prevention of prostate cancer: a systematic review and meta-analysis. Cancer Causes Control. 2005;16(9):1125–1131. doi: 10.1007/s10552-005-0334-2. [PubMed] [CrossRef] [Google Scholar]
104. Burney PG, Comstock GW, Morris JS. Serologic precursors of cancer: serum micronutrients and the subsequent risk of pancreatic cancer. Am J Clin Nutr. 1989;49(5):895–900. doi: 10.1093/ajcn/49.5.895. [PubMed] [CrossRef] [Google Scholar]
105. Murawaki Y, Tsuchiya H, Kanbe T, Harada K, Yashima K, Nozaka K, Tanida O. et al. Aberrant expression of selenoproteins in the progression of colorectal cancer. Cancer Lett. 2008;259(2):218–230. doi: 10.1016/j.canlet.2007.10.019. [PubMed] [CrossRef] [Google Scholar]
106. Jackson MI, Combs GF Jr. Selenium and anticarcinogenesis: underlying mechanisms. Curr Opin Clin Nutr Metab Care. 2008;11(6):718–726. doi: 10.1097/MCO.0b013e3283139674. [PubMed] [CrossRef] [Google Scholar]
107. Smith ML, Lancia JK, Mercer TI, Ip C. Selenium compounds regulate p53 by common and distinctive mechanisms. Anticancer Res. 2004;24(3a):1401–1408. [PubMed] [Google Scholar]
108. Fowler BA, Whittaker MH, Lipsky M, Wang G, Chen XQ. Oxidative stress induced by lead, cadmium and arsenic mixtures: 30-day, 90-day, and 180-day drinking water studies in rats: an overview. Biometals. 2004;17(5):567–568. doi: 10.1023/B:BIOM.0000045740.52182.9d. [PubMed] [CrossRef] [Google Scholar]
109. Schrauzer GN. Anticarcinogenic effects of selenium. Cell Mol Life Sci. 2000;57(13-14):1864–1873. doi: 10.1007/PL00000668. [PubMed] [CrossRef] [Google Scholar]
110. Brotherton L, Welton M, Robb SW. Racial disparities of pancreatic cancer in Georgia: a county-wide comparison of incidence and mortality across the state, 2000-2011. Cancer Med. 2016;5(1):100–110. doi: 10.1002/cam4.552. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
111. Shavers VL, Harlan LC, Jackson M, Robinson J. Racial/ethnic patterns of care for pancreatic cancer. J Palliat Med. 2009;12(7):623–630. doi: 10.1089/jpm.2009.0036. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
112. Ma J, Siegel R, Jemal A. Pancreatic cancer death rates by race among US men and women, 1970-2009. J Natl Cancer Inst. 2013;105(22):1694–1700. doi: 10.1093/jnci/djt292. [PubMed] [CrossRef] [Google Scholar]
113. American Cancer Society. Cancer Facts and Figures 2014. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2014.html. 2014. Accessed 05 October 2018.
114. Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–1261. doi: 10.1053/j.gastro.2013.01.068. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
115. Arnold LD, Patel AV, Yan Y, Jacobs EJ, Thun MJ, Calle EE, Colditz GA. Are racial disparities in pancreatic cancer explained by smoking and overweight/obesity? Cancer Epidemiol Biomarkers Prev. 2009;18(9):2397–2405. doi: 10.1158/1055-9965.EPI-09-0080. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
116. Pernick NL, Sarkar FH, Philip PA, Arlauskas P, Shields AF, Vaitkevicius VK, Dugan MC. et al. Clinicopathologic analysis of pancreatic adenocarcinoma in African Americans and Caucasians. Pancreas. 2003;26(1):28–32. doi: 10.1097/00006676-200301000-00006. [PubMed] [CrossRef] [Google Scholar]
117. Dong M, Nio Y, Tamura K, Song MM, Guo KJ, Guo RX, Dong YT. Ki-ras point mutation and p53 expression in human pancreatic cancer: a comparative study among Chinese, Japanese, and Western patients. Cancer Epidemiol Biomarkers Prev. 2000;9(3):279–284. [PubMed] [Google Scholar]
118. Song MM, Nio Y, Dong M, Tamura K, Furuse K, Tian YL, He SG. et al. Comparison of K-ras point mutations at codon 12 and p21 expression in pancreatic cancer between Japanese and Chinese patients. J Surg Oncol. 2000;75(3):176–185. doi: 10.1002/1096-9098(200011)75:3<176::AID-JSO5>3.0.CO;2-W. [PubMed] [CrossRef] [Google Scholar]
119. Longnecker DS, Karagas MR, Tosteson TD, Mott LA. Racial differences in pancreatic cancer: comparison of survival and histologic types of pancreatic carcinoma in Asians, blacks, and whites in the United States. Pancreas. 2000;21(4):338–343. doi: 10.1097/00006676-200011000-00003. [PubMed] [CrossRef] [Google Scholar]
120. Batabyal P, Vander Hoorn S, Christophi C, Nikfarjam M. Association of diabetes mellitus and pancreatic adenocarcinoma: a meta-analysis of 88 studies. Ann Surg Oncol. 2014;21(7):2453–2462. doi: 10.1245/s10434-014-3625-6. [PubMed] [CrossRef] [Google Scholar]
121. Pezzilli R, Pagano N. Is diabetes mellitus a risk factor for pancreatic cancer? World J Gastroenterol. 2013;19(30):4861–4866. doi: 10.3748/wjg.v19.i30.4861. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
122. McAuliffe JC, Christein JD. Type 2 diabetes mellitus and pancreatic cancer. Surg Clin North Am. 2013;93(3):619–627. doi: 10.1016/j.suc.2013.02.003. [PubMed] [CrossRef] [Google Scholar]
123. Haugvik SP, Hedenstrom P, Korsaeth E, Valente R, Hayes A, Siuka D, Maisonneuve P. et al. Diabetes, smoking, alcohol use, and family history of cancer as risk factors for pancreatic neuroendocrine tumors: a systematic review and meta-analysis. Neuroendocrinology. 2015;101(2):133–142. doi: 10.1159/000375164. [PubMed] [CrossRef] [Google Scholar]
124. Stevens RJ, Roddam AW, Beral V. Pancreatic cancer in type 1 and young-onset diabetes: systematic review and meta-analysis. Br J Cancer. 2007;96(3):507–509. doi: 10.1038/sj.bjc.6603571. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
125. Rosato V, Polesel J, Bosetti C, Serraino D, Negri E, La Vecchia C. Population attributable risk for pancreatic cancer in Northern Italy. Pancreas. 2015;44(2):216–220. doi: 10.1097/MPA.0000000000000251. [PubMed] [CrossRef] [Google Scholar]
126. Li D, Tang H, Hassan MM, Holly EA, Bracci PM, Silverman DT. Diabetes and risk of pancreatic cancer: a pooled analysis of three large case-control studies. Cancer Causes Control. 2011;22(2):189–197. doi: 10.1007/s10552-010-9686-3. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
127. Liao KF, Lai SW, Li CI, Chen WC. Diabetes mellitus correlates with increased risk of pancreatic cancer: a population-based cohort study in Taiwan. J Gastroenterol Hepatol. 2012;27(4):709–713. doi: 10.1111/j.1440-1746.2011.06938.x. [PubMed] [CrossRef] [Google Scholar]
128. Bosetti C, Rosato V, Li D, Silverman D, Petersen GM, Bracci PM, Neale RE. et al. Diabetes, antidiabetic medications, and pancreatic cancer risk: an analysis from the International Pancreatic Cancer Case-Control Consortium. Ann Oncol. 2014;25(10):2065–2072. doi: 10.1093/annonc/mdu276. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
129. Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnquist HJ, Larsson J. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg. 1993;80(8):1047–1050. doi: 10.1002/bjs.1800800841. [PubMed] [CrossRef] [Google Scholar]
130. Gullo L, Pezzilli R, Morselli-Labate AM, Italian Pancreatic Cancer Study G. Diabetes and the risk of pancreatic cancer. N Engl J Med. 1994;331(2):81–84. doi: 10.1056/NEJM199407143310203. [PubMed] [CrossRef] [Google Scholar]
131. Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995;273(20):1605–1609. doi: 10.1001/jama.1995.03520440059037. [PubMed] [CrossRef] [Google Scholar]
132. Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92(11):2076–2083. doi: 10.1038/sj.bjc.6602619. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
133. Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B. et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25(9):2097–2116. doi: 10.1007/s11095-008-9661-9. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
134. Jacobs EJ, Chanock SJ, Fuchs CS, Lacroix A, McWilliams RR, Steplowski E, Stolzenberg-Solomon RZ. et al. Family history of cancer and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan) Int J Cancer. 2010;127(6):1421–1428. doi: 10.1002/ijc.25148. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
135. Permuth-Wey J, Egan KM. Family history is a significant risk factor for pancreatic cancer: results from a systematic review and meta-analysis. Fam Cancer. 2009;8(2):109–117. doi: 10.1007/s10689-008-9214-8. [PubMed] [CrossRef] [Google Scholar]
136. Shi C, Hruban RH, Klein AP. Familial pancreatic cancer. Arch Pathol Lab Med. 2009;133(3):365–374. [PubMed] [Google Scholar]
137. Greer JB, Whitcomb DC, Brand RE. Genetic predisposition to pancreatic cancer: a brief review. Am J Gastroenterol. 2007;102(11):2564–2569. doi: 10.1111/j.1572-0241.2007.01475.x. [PubMed] [CrossRef] [Google Scholar]
138. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–620. doi: 10.1016/S0140-6736(10)62307-0. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
139. Brune KA, Lau B, Palmisano E, Canto M, Goggins MG, Hruban RH, Klein AP. Importance of age of onset in pancreatic cancer kindreds. J Natl Cancer Inst. 2010;102(2):119–126. doi: 10.1093/jnci/djp466. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
140. Wang L, Brune KA, Visvanathan K, Laheru D, Herman J, Wolfgang C, Schulick R. et al. Elevated cancer mortality in the relatives of patients with pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2829–2834. doi: 10.1158/1055-9965.EPI-09-0557. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
141. Ghiorzo P. Genetic predisposition to pancreatic cancer. World J Gastroenterol. 2014;20(31):10778–10789. doi: 10.3748/wjg.v20.i31.10778. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
142. Shi C, Daniels JA, Hruban RH. Molecular characterization of pancreatic neoplasms. Adv Anat Pathol. 2008;15(4):185–195. doi: 10.1097/PAP.0b013e31817bf57d. [PubMed] [CrossRef] [Google Scholar]
143. Solomon S, Das S, Brand R, Whitcomb DC. Inherited pancreatic cancer syndromes. Cancer J. 2012;18(6):485–491. doi: 10.1097/PPO.0b013e318278c4a6. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
144. Murphy KM, Brune KA, Griffin C, Sollenberger JE, Petersen GM, Bansal R, Hruban RH. et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17% Cancer Res. 2002;62(13):3789–3793. [PubMed] [Google Scholar]
145. Hahn SA, Greenhalf B, Ellis I, Sina-Frey M, Rieder H, Korte B, Gerdes B. et al. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003;95(3):214–221. doi: 10.1093/jnci/95.3.214. [PubMed] [CrossRef] [Google Scholar]
146. Couch FJ, Johnson MR, Rabe KG, Brune K, de Andrade M, Goggins M, Rothenmund H. et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(2):342–346. doi: 10.1158/1055-9965.EPI-06-0783. [PubMed] [CrossRef] [Google Scholar]
147. Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, Lin JC. et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324(5924):217. doi: 10.1126/science.1171202. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
148. Slater EP, Langer P, Niemczyk E, Strauch K, Butler J, Habbe N, Neoptolemos JP. et al. PALB2 mutations in European familial pancreatic cancer families. Clin Genet. 2010;78(5):490–494. doi: 10.1111/j.1399-0004.2010.01425.x. [PubMed] [CrossRef] [Google Scholar]
149. Tischkowitz MD, Sabbaghian N, Hamel N, Borgida A, Rosner C, Taherian N, Srivastava A. et al. Analysis of the gene coding for the BRCA2-interacting protein PALB2 in familial and sporadic pancreatic cancer. Gastroenterology. 2009;137(3):1183–1186. doi: 10.1053/j.gastro.2009.06.055. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
150. Klein AP. Genetic susceptibility to pancreatic cancer. Mol Carcinog. 2012;51(1):14–24. doi: 10.1002/mc.20855. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
151. Risch HA, Yu H, Lu L, Kidd MS. ABO blood group, Helicobacter pylori seropositivity, and risk of pancreatic cancer: a case-control study. J Natl Cancer Inst. 2010;102(7):502–505. doi: 10.1093/jnci/djq007. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
152. Risch HA, Lu L, Kidd MS, Wang J, Zhang W, Ni Q, Gao YT. et al. Helicobacter pylori seropositivities and risk of pancreatic carcinoma. Cancer Epidemiol Biomarkers Prev. 2014;23(1):172–178. doi: 10.1158/1055-9965.EPI-13-0447. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
153. Risch HA. Etiology of pancreatic cancer, with a hypothesis concerning the role of N-nitroso compounds and excess gastric acidity. J Natl Cancer Inst. 2003;95(13):948–960. doi: 10.1093/jnci/95.13.948. [PubMed] [CrossRef] [Google Scholar]
154. Chen XZ, Schottker B, Castro FA, Chen H, Zhang Y, Holleczek B, Brenner H. Association of helicobacter pylori infection and chronic atrophic gastritis with risk of colonic, pancreatic and gastric cancer: A ten-year follow-up of the ESTHER cohort study. Oncotarget. 2016;7(13):17182–17193. doi: 10.18632/oncotarget.7946. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
155. Ben Q, Wang K, Yuan Y, Li Z. Pancreatic cancer incidence and outcome in relation to ABO blood groups among Han Chinese patients: a case-control study. Int J Cancer. 2011;128(5):1179–1186. doi: 10.1002/ijc.25426. [PubMed] [CrossRef] [Google Scholar]
156. Anstee DJ. The relationship between blood groups and disease. Blood. 2010;115(23):4635–4643. doi: 10.1182/blood-2010-01-261859. [PubMed] [CrossRef] [Google Scholar]
157. Liumbruno GM, Franchini M. Beyond immunohaematology: the role of the ABO blood group in human diseases. Blood Transfus. 2013;11(4):491–499. [PMC free article] [PubMed] [Google Scholar]
158. Franchini M, Favaloro EJ, Targher G, Lippi G. ABO blood group, hypercoagulability, and cardiovascular and cancer risk. Crit Rev Clin Lab Sci. 2012;49(4):137–149. doi: 10.3109/10408363.2012.708647. [PubMed] [CrossRef] [Google Scholar]
159. Franchini M, Liumbruno GM, Lippi G. The prognostic value of ABO blood group in cancer patients. Blood Transfus. 2016;14(5):434–440. [PMC free article] [PubMed] [Google Scholar]
160. Zhang BL, He N, Huang YB, Song FJ, Chen KX. ABO blood groups and risk of cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15(11):4643–4650. doi: 10.7314/APJCP.2014.15.11.4643. [PubMed] [CrossRef] [Google Scholar]
161. Macafee AL. ABO blood groups and carcinoma of pancreas. Ulster Med J. 1964;33(2):129–131. [PMC free article] [PubMed] [Google Scholar]
162. Vioque J, Walker AM. [Pancreatic cancer and ABO blood types: a study of cases and controls] Med Clin (Barc) 1991;96(20):761–764. [PubMed] [Google Scholar]
163. Annese V, Minervini M, Gabbrielli A, Gambassi G, Manna R. ABO blood groups and cancer of the pancreas. Int J Pancreatol. 1990;6(2):81–88. [PubMed] [Google Scholar]
164. Wolpin BM, Chan AT, Hartge P, Chanock SJ, Kraft P, Hunter DJ, Giovannucci EL. et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101(6):424–431. doi: 10.1093/jnci/djp020. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
165. Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA, Bueno-de-Mesquita HB. et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009;41(9):986–990. doi: 10.1038/ng.429. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
166. Rizzato C, Campa D, Giese N, Werner J, Rachakonda PS, Kumar R, Schanne M. et al. Pancreatic cancer susceptibility loci and their role in survival. PLoS One. 2011;6(11):e27921. doi: 10.1371/journal.pone.0027921. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
167. Dandona M, Gao F, Linehan DC, Wang-Gillam A. Re: ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2010;102(2):135–137. doi: 10.1093/jnci/djp447. author reply 137. [PubMed] [CrossRef] [Google Scholar]
168. Rahbari NN, Bork U, Hinz U, Leo A, Kirchberg J, Koch M, Buchler MW. et al. AB0 blood group and prognosis in patients with pancreatic cancer. BMC Cancer. 2012;12:319. [PMC free article] [PubMed] [Google Scholar]
169. Wang DS, Wang ZQ, Zhang L, Qiu MZ, Luo HY, Ren C, Zhang DS. et al. Are risk factors associated with outcomes in pancreatic cancer? PLoS One. 2012;7(7):e41984. doi: 10.1371/journal.pone.0041984. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
170. Hakomori S. Antigen structure and genetic basis of histo-blood groups A, B and O: their changes associated with human cancer. Biochim Biophys Acta. 1999;1473(1):247–266. doi: 10.1016/S0304-4165(99)00183-X. [PubMed] [CrossRef] [Google Scholar]
171. Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120(3):682–707. doi: 10.1053/gast.2001.22586. [PubMed] [CrossRef] [Google Scholar]
172. Ekbom A, McLaughlin JK, Karlsson BM, Nyren O, Gridley G, Adami HO, Fraumeni JF Jr. Pancreatitis and pancreatic cancer: a population-based study. J Natl Cancer Inst. 1994;86(8):625–627. doi: 10.1093/jnci/86.8.625. [PubMed] [CrossRef] [Google Scholar]
173. Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24(3):349–358. doi: 10.1016/j.bpg.2010.02.007. [PubMed] [CrossRef] [Google Scholar]
174. Duell EJ, Lucenteforte E, Olson SH, Bracci PM, Li D, Risch HA, Silverman DT. et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4) Ann Oncol. 2012;23(11):2964–2970. doi: 10.1093/annonc/mds140. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
175. Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol. 2010;7(3):131–145. doi: 10.1038/nrgastro.2010.6. [PubMed] [CrossRef] [Google Scholar]
176. Hirota M, Shimosegawa T, Masamune A, Kikuta K, Kume K, Hamada S, Kihara Y. et al. The sixth nationwide epidemiological survey of chronic pancreatitis in Japan. Pancreatology. 2012;12(2):79–84. doi: 10.1016/j.pan.2012.02.005. [PubMed] [CrossRef] [Google Scholar]
177. Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP. et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328(20):1433–1437. doi: 10.1056/NEJM199305203282001. [PubMed] [CrossRef] [Google Scholar]
178. Bracci PM, Wang F, Hassan MM, Gupta S, Li D, Holly EA. Pancreatitis and pancreatic cancer in two large pooled case-control studies. Cancer Causes Control. 2009;20(9):1723–1731. doi: 10.1007/s10552-009-9424-x. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
179. Olson SH. Selected medical conditions and risk of pancreatic cancer. Mol Carcinog. 2012;51(1):75–97. doi: 10.1002/mc.20816. [PubMed] [CrossRef] [Google Scholar]
180. Midha S, Sreenivas V, Kabra M, Chattopadhyay TK, Joshi YK, Garg PK. Genetically determined chronic pancreatitis but not alcoholic pancreatitis is a strong risk factor for pancreatic cancer. Pancreas. 2016;45(10):1478–1484. doi: 10.1097/MPA.0000000000000684. [PubMed] [CrossRef] [Google Scholar]
181. Whitcomb DC. Inflammation and Cancer V. Chronic pancreatitis and pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2004;287(2):G315–319. doi: 10.1152/ajpgi.00115.2004. [PubMed] [CrossRef] [Google Scholar]
182. Lowenfels AB, Maisonneuve P, Whitcomb DC. Risk factors for cancer in hereditary pancreatitis. International Hereditary Pancreatitis Study Group. Med Clin North Am. 2000;84(3):565–573. doi: 10.1016/S0025-7125(05)70240-6. [PubMed] [CrossRef] [Google Scholar]
183. Howes N, Lerch MM, Greenhalf W, Stocken DD, Ellis I, Simon P, Truninger K. et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004;2(3):252–261. doi: 10.1016/S1542-3565(04)00013-8. [PubMed] [CrossRef] [Google Scholar]
184. Raimondi S, Maisonneuve P, Lohr JM, Lowenfels AB. Early onset pancreatic cancer: evidence of a major role for smoking and genetic factors. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1894–1897. doi: 10.1158/1055-9965.EPI-07-0341. [PubMed] [CrossRef] [Google Scholar]
185. Lowenfels AB, Maisonneuve P, DiMagno EP, Elitsur Y, Gates LK Jr, Perrault J, Whitcomb DC. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89(6):442–446. doi: 10.1093/jnci/89.6.442. [PubMed] [CrossRef] [Google Scholar]
186. Lowenfels AB, Maisonneuve P, Whitcomb DC, Lerch MM, DiMagno EP. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001;286(2):169–170. doi: 10.1001/jama.286.2.169. [PubMed] [CrossRef] [Google Scholar]
187. Greenhalf W, Grocock C, Harcus M, Neoptolemos J. Screening of high-risk families for pancreatic cancer. Pancreatology. 2009;9(3):215–222. doi: 10.1159/000210262. [PubMed] [CrossRef] [Google Scholar]
188. Shin EJ, Canto MI. Pancreatic cancer screening. Gastroenterol Clin North Am. 2012;41(1):143–157. doi: 10.1016/j.gtc.2011.12.001. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
189. Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. doi: 10.1056/NEJMra0901557. [PubMed] [CrossRef] [Google Scholar]
190. Cappelli G, Paladini S, D’Agata A. [Tumor markers in the diagnosis of pancreatic cancer] Tumori. 1999;85(1 Suppl 1):S19–21. doi: 10.1177/030089169908501s06. [PubMed] [CrossRef] [Google Scholar]
191. Walsh RM, Vogt DP, Henderson JM, Hirose K, Mason T, Bencsath K, Hammel J. et al. Management of suspected pancreatic cystic neoplasms based on cyst size. Surgery. 2008;144(4):677–684. discussion 684-675. [PubMed] [Google Scholar]
192. Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W. et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12(3):183–197. doi: 10.1016/j.pan.2012.04.004. [PubMed] [CrossRef] [Google Scholar]
193. Canto MI, Goggins M, Yeo CJ, Griffin C, Axilbund JE, Brune K, Ali SZ. et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004;2(7):606–621. doi: 10.1016/S1542-3565(04)00244-7. [PubMed] [CrossRef] [Google Scholar]
194. Vitone LJ, Greenhalf W, Howes NR, Neoptolemos JP. Hereditary pancreatitis and secondary screening for early pancreatic cancer. Rocz Akad Med Bialymst. 2005;50:73–84. [PubMed] [Google Scholar]
195. American gastroenterological association medical position statement: epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. Gastroenterology. 1999;117(6):1463–1484. doi: 10.1016/S0016-5085(99)70297-0. [PubMed] [CrossRef] [Google Scholar]
196. Krech RL, Walsh D. Symptoms of pancreatic cancer. J Pain Symptom Manage. 1991;6(6):360–367. doi: 10.1016/0885-3924(91)90027-2. [PubMed] [CrossRef] [Google Scholar]
197. Klauss M, Schobinger M, Wolf I, Werner J, Meinzer HP, Kauczor HU, Grenacher L. Value of three-dimensional reconstructions in pancreatic carcinoma using multidetector CT: initial results. World J Gastroenterol. 2009;15(46):5827–5832. doi: 10.3748/wjg.15.5827. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
198. Wong JC, Lu DS. Staging of pancreatic adenocarcinoma by imaging studies. Clin Gastroenterol Hepatol. 2008;6(12):1301–1308. doi: 10.1016/j.cgh.2008.09.014. [PubMed] [CrossRef] [Google Scholar]
199. Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97(6):1386–1391. doi: 10.1111/j.1572-0241.2002.05777.x. [PubMed] [CrossRef] [Google Scholar]
200. Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, Benson AB 3rd. et al. Pancreatic adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(8):1028–1061. doi: 10.6004/jnccn.2017.0131. [PubMed] [CrossRef] [Google Scholar]
201. Safi F, Roscher R, Bittner R, Schenkluhn B, Dopfer HP, Beger HG. High sensitivity and specificity of CA 19-9 for pancreatic carcinoma in comparison to chronic pancreatitis. Serological and immunohistochemical findings. Pancreas. 1987;2(4):398–403. doi: 10.1097/00006676-198707000-00006. [PubMed] [CrossRef] [Google Scholar]
202 .Ilic I, Ilic M. International patterns in incidence and mortality trends of pancreatic cancer in the last three decades: A joinpoint regression analysis. World J Gastroenterol. 2022 Aug 28;28(32):4698-4715. doi: 10.3748/wjg.v28.i32.4698. PMID: 36157927; PMCID: PMC9476884.